What is the formula for calculating molarity?
Molarity Formula: The equation for calculating molarity is the ratio of the moles of solute whose molarity is to be calculated and the volume of solvent used to dissolve the given solute. \(M = \frac{n}{V}\) Here, M is the molality of the solution that is to be calculated. n is the number of moles of the solute
What is the bottom unit of molarity in this equation?
Jan 27, 2022 · The two most commonly used concentration units are Molarity and morality. The Molarity formula is expressed as M o l a r i t y ( M) = M o l e s o f S o l u t e V o l u m e o f S o l u t i o n ( i n L). Its unit is m o l / L. Furthermore, Molarity relies on the volume of a solution expressed in litres and not the volume of solvent.
What is molality of a solution?
The formula of titration is= Molarity of acid(M 1) × Volume of acid(V 1) = Molarity of the base(M 1) × Volume of the base(V 2) 3. What is the major difference between Molarity and molality?
What is the molarity of a mixture?
Step Transfer the sodium chloride to a clean, dry flask. Step Add water to the until the total volume of the solution is . Step Stir until the is completely dissolved. The accuracy of our molar concentration depends on our choice of glassware, as well as the accuracy of the balance we use to measure out the solute.
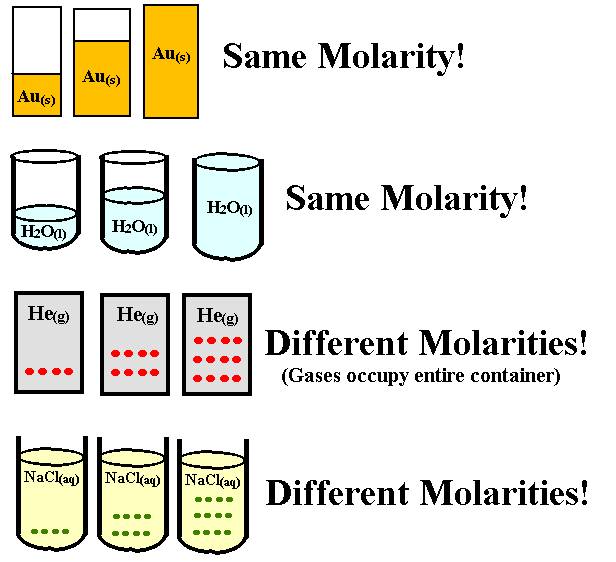
What is the formula to calculate molarity?
As mass / volume = molarity * molar mass , then mass / (volume * molar mass) = molarity . Substitute the known values to calculate the molarity: molarity = 5 / (1.2 * 36.46) = 0.114 mol/l = 0.114 M . You can also use this molarity calculator to find the mass concentration or molar mass.Mar 14, 2022
What is molarity with example?
Molarity=moles of solutelitres of solution. For example, a 0.25 mol/L NaOH solution contains 0.25 mol of sodium hydroxide in every litre of solution. To calculate the molarity of a solution, you need to know the number of moles of solute and the total volume of the solution.May 22, 2014
How do you find the molarity of NaCl?
One mole of sodium (Na) is 22.99 g, and 1 mole of chlorine is 35.45 g. For sodium chloride (NaCl) they are in a ratio of 1:1 so the molar mass of NaCl is 22.99 + 35.45 = 58.44 g/mol.
What do you understand by molarity write with formula?
Molarity is the ratio of the moles of a solute to the total liters of a solution. The solution includes both the solute and the solvent....Molarity vs molality.Molarity (M)Molality (m)UnitsMmEquationM = moles solute / liters solutionm = moles solute / kg solventRatio of moles to:Volume (in liters)Mass (in kilograms)2 more rows•Apr 29, 2020
How do you find final molarity?
Calculate the final molarity of the mixed solution using the equation molarity = moles ÷ liter. For the example, the final molarity is 0.0135 moles ÷ 0.170 liters = 0.079 M.Apr 30, 2018
What is the molarity of cacl2?
Molarity is 2.0M, so the number of moles is 0.125 x 2 = 0.25 mol. Now simply multiply this by the molar mass of calcium chloride, which is 110.98 g/mol, giving you the answer 27.745 g.
What is the molarity of NaOH?
The molarity of NaOH is 2.002 x 10 -2 moles x 1000 mL/L / 25.0 mL = 0.801 Molar.Dec 11, 2020
1. How do you find liters from Molarity?
In order to find liters from Molarity, we need to follow the following steps:You need to find the volume of the solution in liters List out the thi...
2. How do you calculate Molarity using the concept of titration?
In order to find the concentration out of the given molarity of a solution,you need to follow some simple basic steps:Use the normal titration form...
3. What is the major difference between Molarity and molality?
The most important criteria which differentiate molality and molarity from each other is the difference between the solution and the solvent .Molar...
4. How do you calculate molality from Molarity?
In order to calculate molality from Molarity,the following steps are necessary to follow:Make an assumption. Let us suppose you have 1L of the solu...
5. What is the difference between moles and Molarity
Both moles and Molarity are measuring substances. The major difference between the moles and Molarity is , The measurement of the number of substan...
6. Why is the volume of a solid solute not considered while calculating molarity?
To answer this question, you will have to use the formula of Molarity and your previous knowledge of the solution. You know that when a solid is pe...
7. How can you determine the Molarity equation?
If you understand the definition of molarity in this section, you will find the meaning of all terms associated to form the equation. Remember the...
1. Define the term Molarity.
Molarity (M) also known as Molar Concentration is a technique that is used to calculate the concentration of a solute that is present in a definite...
2. Define the term Molality.
Molality (m) is a technique that is used to calculate the concentration of a solute that is present in a particular mass of the solvent. The solute...
3. What is the difference between Molarity and Molality?
Molarity(M) is the method of calculating the concentration of a solute within the solution - that is expressed in the unit liters - it is contained...
4. Define the term Mole Fraction.
Mole Fraction, also known as Molar Fraction is the calculation of the number of moles of one particular constituent divided by the total amount of...
5. Define the term Weight Percentage
Weight Percentage, also known as the Mass Percentage, can be defined as the ratio of the mass of a solute and the mass of the solution multiplied b...
6. Why is the volume of a solid solute not considered while calculating molarity?
To answer this question, you will have to use the formula of molarity and your previous knowledge of the solution. You know that when a solid is pe...
7. How can you determine the Molarity equation?
If you understand the definition of molarity in this section, you will find the meaning of all terms associated to form the equation. Remember the...
Popular Posts:
- 1. blackboard box grading group comments
- 2. blackboard grade complete/incomplete
- 3. blackboard assignment tool allows the student to attach multiple file to one assignment
- 4. mmunialty blackboard
- 5. how can i post publisher's slides on blackboard without violating copyright
- 6. blackboard see which graders are assigned
- 7. how to indent a paragraph in blackboard
- 8. how to make annoucement tab landing in blackboard
- 9. how to link cengage to blackboard for student
- 10. the guest what was written on the blackboard